Every atom tells a story. Some are satisfied just as they are. Others are always looking for something more—especially a missing piece to feel complete. In the world of chemistry, this search for balance is often guided by a concept known as electron affinity. While it might sound technical, it’s actually quite a simple and fascinating idea: it’s all about how much an atom wants to gain an electron.
In this article, we’ll explore what electron affinity really means, how it works, and why it plays a key role in the chemistry of life, materials, and technology.
The Story of a Hungry Atom
Let’s imagine atoms as characters in a story.
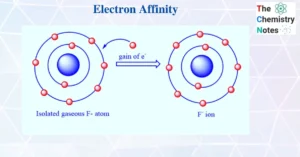
Think of fluorine as a little puzzle piece that’s almost finished—except for one empty slot. It searches and searches for just one more piece to complete its outer shell. Then it finds a spare electron. When that electron joins fluorine, it fits perfectly. Fluorine is happy. But more than that—it releases energy to show how satisfied it is. That release of energy is what we call electron affinity.
Electron affinity is a way of measuring how eager an atom is to gain an electron. The more energy it releases, the stronger its desire.
What Is Electron Affinity in Simple Words?
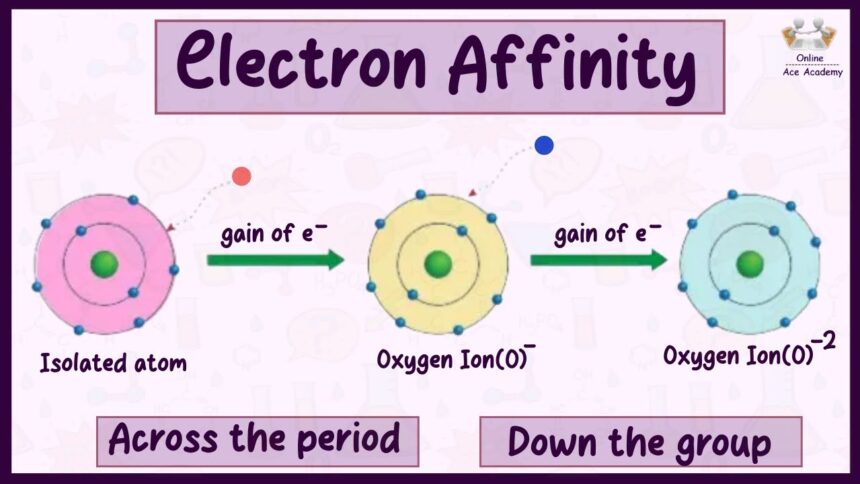
Electron affinity is the energy change that happens when an atom gains an electron.
-
If the atom releases energy, it means it really wanted that electron.
-
If the atom absorbs energy, it means it didn’t want the electron but was forced to take it.
Imagine giving a gift to someone:
-
Some people are thrilled and say, “Wow, thank you!” That’s like an atom with high electron affinity.
-
Others may politely accept it but don’t really care. That’s like an atom with low electron affinity.
In chemistry, atoms behave similarly. Some welcome the new electron and become more stable. Others resist it.
Why Is Electron Affinity Important?
Understanding electron affinity helps explain many things in chemistry:
-
Why certain atoms are more reactive than others.
-
Why some elements form strong bonds.
-
Why elements like oxygen and chlorine are often found in reactions that release energy.
Electron affinity is one of the driving forces behind chemical bonding—how and why atoms join to create everything from water to DNA.
Electron Affinity and the Periodic Table
If you take a close look at the periodic table, you’ll find some helpful patterns.
General Trends:
-
Across a period (left to right): Electron affinity increases.
-
Down a group (top to bottom): Electron affinity decreases.
Why? Because as you move across a period, atoms get closer to having a full outer shell. They’re eager to complete it—so they “want” that extra electron more.
As you go down a group, atoms get bigger. The outer electrons are farther from the nucleus and feel less pull. That makes them less interested in taking in new electrons.
Meet the Characters: Elements with High and Low Electron Affinity
Fluorine and Chlorine
These are stars of high electron affinity. Just one more electron and they complete their outer shell. When they gain it, they release a lot of energy. This makes them highly reactive—always ready to bond.
Noble Gases
Then there are the quiet, content elements—helium, neon, argon. Their outer shells are already full. They don’t need any more electrons. In fact, forcing them to accept an electron would cost energy, not release it. That’s why noble gases have zero or even negative electron affinity.
Real-Life Analogy: A Job Opening
Think of an atom as a company.
-
Fluorine is a company that has one position left. When it finds the right candidate (electron), it throws a celebration—releasing energy.
-
Neon is a company that has no open positions. If someone still shows up, the company has to build a new office space just to fit them—spending energy instead of gaining it.
This explains why some atoms are eager for electrons, while others are not.
Electron Affinity and Bonding Behavior
Here’s where the story gets practical. Let’s look at a common salt: sodium chloride (NaCl).
-
Sodium (Na) has one extra electron that it wants to give away.
-
Chlorine (Cl) desperately wants one more electron to complete its shell.
When they meet, sodium donates its electron to chlorine. Chlorine is thrilled—it gains stability and releases energy in the process. That energy comes from its electron affinity.
This reaction is the reason we have table salt. It’s also an example of how electron affinity influences real-world chemistry.
Why Electron Affinity Isn’t Always Predictable
Even though the periodic table gives us trends, electron affinity doesn’t always follow neat rules. Here’s why:
-
Some atoms have half-filled or full sublevels in their electron shells. Gaining an extra electron may disturb their stability, so they resist it.
-
For example, nitrogen has a half-filled p orbital. Adding one more electron causes repulsion between electrons, so its electron affinity is lower than expected.
So while trends are helpful, the exact value of electron affinity depends on an atom’s electron structure.
Beyond the Lab: Where We See Electron Affinity in Action
You might think electron affinity only matters to scientists, but it affects everyday life in powerful ways:
-
Batteries: The movement of electrons from one material to another (based on their electron affinities) powers your phone.
-
Pollution control: Chemical reactions used to trap harmful gases rely on how certain atoms attract electrons.
-
Medicine: The way drugs interact with molecules in the body depends on how atoms accept or reject electrons.
Even the screen you’re reading this on works thanks to materials selected based on their electron affinity!
Conclusion: A Small Force with Big Impact
Electron affinity may sound like a small thing—it’s just about atoms gaining electrons. But behind that small act lies a powerful truth about chemistry: everything seeks balance. Whether it’s fluorine trying to complete its shell or sodium looking to give one away, the exchange of electrons is how the world stays in motion.
Understanding electron affinity helps us see why atoms behave the way they do, why bonds form, and why some reactions light up our world—literally.
So next time you think of atoms, remember: even the tiniest particles are driven by a quiet force—a desire for wholeness, for stability, and for connection.